Papers by Dr Peter Michael Moyle
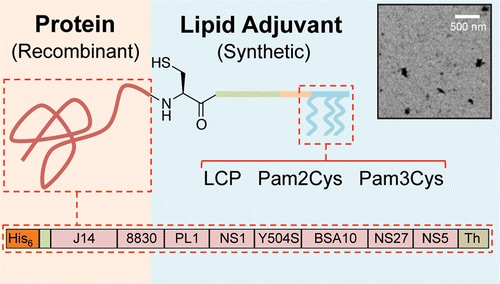
2.1 Site-specific incorporation of three Toll-like receptor 2 targeting adjuvants into semisynthetic, molecularly defined nanoparticles: application to group A streptococcal vaccines
Citation: Moyle PM, Dai W, Zhang Y, Batzloff MR, Good MF, Toth I. Site-specific incorporation of three Toll-like receptor 2 targeting adjuvants into semisynthetic, molecularly defined nanoparticles: application to group A streptococcal vaccines. Bioconjugate Chemistry 2014;25(5):965-78
DOI: 10.1021/bc500108b
Synopsis: This article describes our Expressed Protein Ligation (EPL)-based method to enable the C-terminal specific conjugation of three lipid-based TLR2 ligands {lipid core peptide (LCP), Pam2Cys, Pam3Cys] onto engineered protein antigens as potent immunostimulatory adjuvants. Using this approach we produced a broadly protective and population targeted vaccine against Group A streptococcus. These vaccines self-assembled into nanoparticles in PBS, which exhibited self-adjuvanting properties. The antibodies that were elicited following the immunisation of C57BL6/J mice were able to bind to the surface of group A streptococcal strains that are among the top 20 circulating strains, and are associated with clinical disease.
Notes: This article describes one of our platform technologies for the site-specific attachment of lipid-based adjuvants onto recombinant protein antigens.
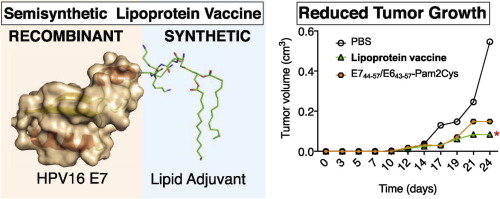
2.2 Combined synthetic and recombinant techniques for the development of lipoprotein-based, self-adjuvanting vaccines targeting human papillomavirus type-16 associated tumors
Citation: Moyle PM, Dai W, Liu T-Y, Hussein WM, Maruthayanar P, Wells JW, McMillan NAJ, Skwarczynski M, Toth I. Combined synthetic and recombinant techniques for the development of lipoprotein-based, self-adjuvanting vaccines targeting human papillomavirus type-16 associated tumors. Bioorganic & Medicinal Chemistry Letters 2015;25(23):5570-5
DOI: 10.1016/j.bmcl.2015.10.049
Synopsis: This article describes the ‘development of a process to enable the production of semisynthetic vaccines based on the site-specific attachment of synthetic bacterial lipid analogs (e.g., Pam2Cys) to a non-oncogenic mutant HPV16 E7 protein to generate molecularly defined vaccines. Many cytotoxic lymphocyte (CTL) epitopes from E7 are delivered by this approach; potentially ensuring that large numbers of immunized individuals can generate CTLs to clear HPV infected cells. Delivery of this construct reduced the growth of HPV16-associated tumors in a TC1 mouse model, the effects of which were better than the potent CTL epitope HPV16 E7(44–57) administered with Montanide ISA51 adjuvant’.
Notes: This article demonstrates the capacity to use our platform technology for the incorporation of Pam2Cys into recombinant vaccines to treat human papillomavirus type-16 (HPV-16) associated cervical cancer.
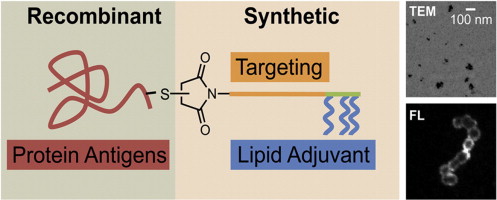
2.3 An efficient, chemically-defined semisynthetic lipid-adjuvanted nanoparticulate vaccine development system
Citation: Moyle PM, Hartas J, Henningham A, Batzlof MR, Good MF, Toth I. An efficient, chemically-defined semisynthetic lipid-adjuvanted nanoparticulate vaccine development system. Nanomedicine Nanotechnology Biology and Medicine 2013;9(7):935-44
DOI: 10.1016/j.nano.2013.01.009
Synopsis: This article describes our development of a novel platform for the development of vaccines against group A streptococcus (Streptococcus pyogenes), consisting of a linear recombinant polytope antigen, incorporating seven S. pyogenes M protein strain-specific antigens and a conserved M protein antigen (J14). A maleimide conjugation approach was developed to enable the site-specific attachment of a lipoamino acid based adjuvant (lipid core peptide; LCP) with this antigen. The produced vaccine could elicit systemic antigen-specific IgG antibodies against all eight antigens without the need for any additional adjuvant. Where a dendritic cell (DC) targeting sequence (DCpep) was incorporated into these vaccines, a higher antibody titer was observed against most antigens.