Papers by Dr Peter Michael Moyle
4.1 Method for the synthesis of mono-ADP-ribose conjugated peptides
Type: Original Research
Citation: Moyle PM, Muir TW. Method for the synthesis of mono-ADP-ribose conjugated peptides. Journal of the American Chemical Society 2010;132(45):15878-80
DOI: 10.1021/ja1064312
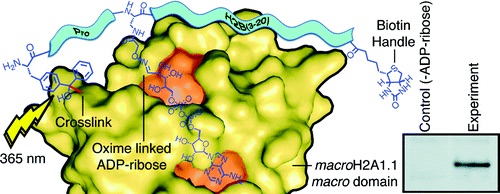
Synopsis: ADP-ribosylation is an important post-translational modification (PTM) associated with DNA damage repair and cell death, and is one of the most difficult PTMs to study. It exists as a monomer, and linear or branched polymers, that can be attached through the side chain of various amino acids via chemical or enzymatic means. Due to the metabolic and chemical instability of this modification, the capacity to purify large amounts of site-specifically modified ADP-ribosylated peptides and proteins to study its role is limited. This paper provides a simple means to incorporate ADP-ribose into peptides (and potentially proteins), and have shown that the incorporation of photocrosslinkers enables us to more efficiently pull-down proteins that interact with this modification. We have also demonstrated that our mono-ADP-ribosylated peptides can serve as substrates for polymerisation by PARP enzymes, and that mono-ADP-ribosylated histone H2B E2 can interact with PARP9. The tools provided in this paper should prove useful for studying ADP-ribosylation.
Note: This original research article was published in the prestigious chemistry journal JACS (the Journal of the American Chemical Society).