Papers by Dr Peter Michael Moyle
1.1 Bioconjugation Approaches to Produce Subunit Vaccines Composed of Protein or Peptide Antigens and Covalently Attached Toll-Like Receptor Ligands
Citation: Zhenghui X, Moyle PM. Bioconjugation approaches to produce subunit vaccines composed of protein or peptide antigens and covalently attached Toll-like receptor ligands. Bioconjugate Chemistry 2017;(in press)
DOI: 10.1021/acs.bioconjchem.7b00478
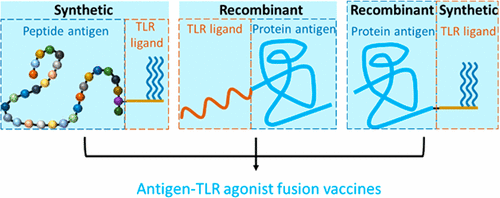
Synopsis: This article reviews approaches that can be used for the conjugation of immunostimulatory adjuvants onto protein antigens in order to improve subunit vaccine potency and safety, as well as to tailor the types of immune responses that are elicited. This work focuses on recombinant (fusion proteins), synthetic (chemical ligation and synthetic peptides) and semisynthetic techniques (e.g. enzyme-mediated ligation reactions).
1.2 Biotechnology Approaches to Produce Potent, Self-Adjuvanting Antigen-Adjuvant Fusion Protein Subunit Vaccines
Citation: Moyle PM. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnology Advances 2017;(in press)
DOI: 10.1016/j.biotechadv.2017.03.005
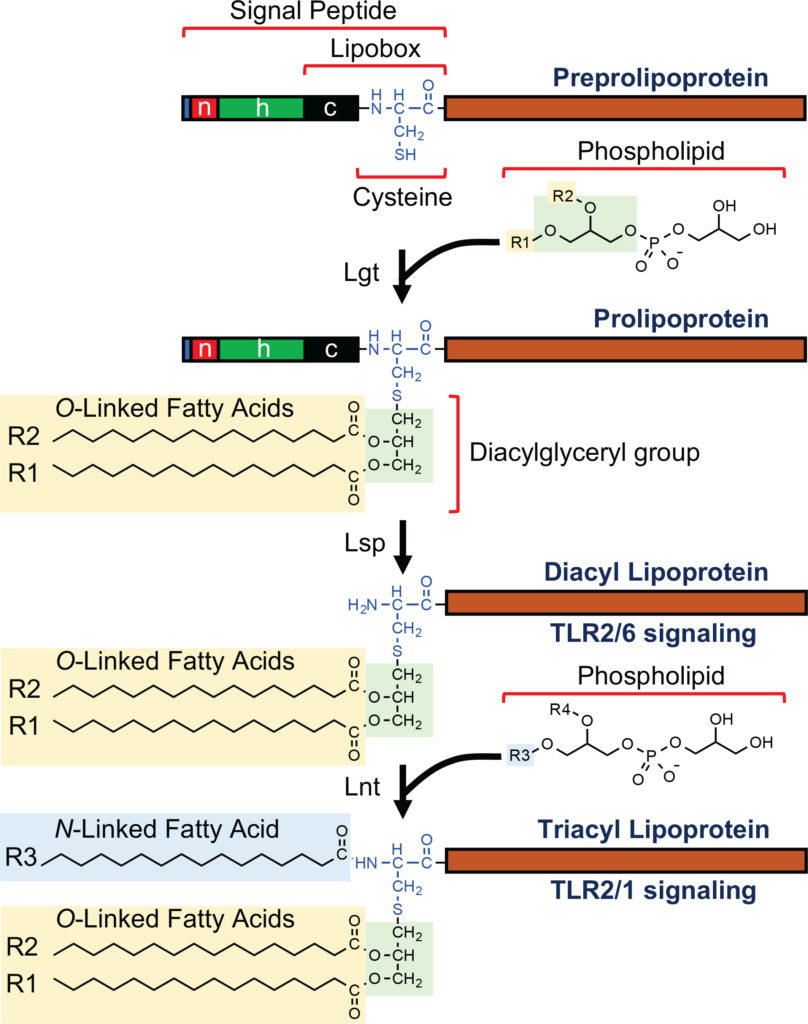
Synopsis: This article reviews four protein-based adjuvants (flagellin, bacterial lipoproteins, the extra domain A of fibronectin (EDA), and heat shock proteins (Hsps)) that can be genetically fused to antigens to enable the recombinant production of antigen-adjuvant fusion proteins, including information on their mechanisms of action, structural or sequence requirements for activity, sequence modifications that enhance their activity or simplify production, adverse effects, and examples of such vaccines in preclinical or human clinical trials.
1.3 Progress in Vaccine Development
Citation: Moyle PM. Progress in Vaccine Development. Current Protocols in Microbiology 2015;36:18.1.1-26
DOI: 10.1002/9780471729259.mc1801s36
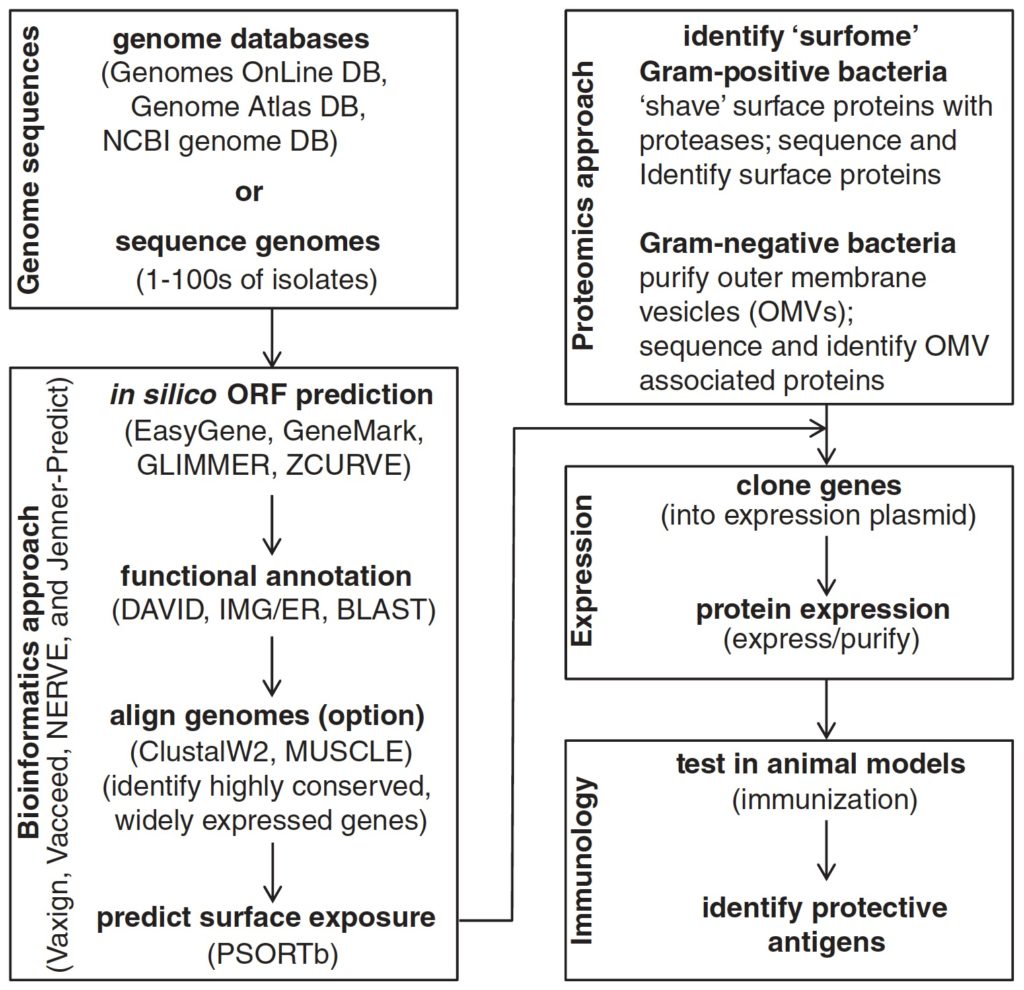
Synopsis: “an overview of the components that are used for the development of modern vaccines including: an introduction to different vaccine types (whole organism, protein/peptide, polysaccharide, conjugate, and DNA vaccines); techniques for identifying subunit antigens; vaccine delivery systems; and immunostimulatory agents (‘adjuvants’), which are fundamental for the development of effective subunit vaccines”.
1.4 Modern subunit vaccines: development, components, and research opportunities
Citation: Moyle PM, Toth I. Modern subunit vaccines: development, components, and research opportunities. ChemMedChem 2013; 8(3):360-76
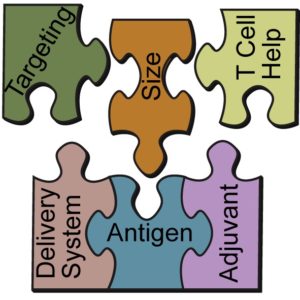
Synopsis: a review of “the current state of the art and future direction in subunit vaccine development, with a focus on” adjuvants, delivery systems, particle size, targeting (e.g. dendritic cells), and “adding components to aid vaccine efficacy in whole immunized populations (e.g. promiscuous T-helper epitopes)”, as well as “their potential to steer the immune response toward a desired response”.
Notes: Highly cited article (> 70 citations 31/7/16, google scholar). ChemMedChem top 10 cited article of 2013. Flagged as ‘highly cited paper’ by Web of Science (placed in the top 1% of the academic field of pharmacology and toxicology).
1.5 Self-adjuvanting lipopeptide vaccines
Citation: Moyle PM, Toth I. Self-adjuvanting lipopeptide vaccines. Curr Med Chem 2008; 15(5):506-16
DOI: 10.2174/092986708783503249
Synopsis: This review “provides an overview of the most studied lipopeptide vaccine systems grouped into the following categories: 1) bacterial lipopeptides, including tri-palmitoyl-S-glyceryl cysteine (Pam3Cys) and di-palmitoyl-S–glyceryl cysteine (Pam2Cys); 2) the lipid-core peptide (LCP) and multiple antigen lipophilic adjuvant carrier (MALAC) systems; 3) single-chain palmitoylated peptides; and 4) glycolipids (e.g. monophosphoryl lipid A). The review also discusses the potential mechanisms of action for lipopeptide and glycolipopeptide vaccines, as well as structure activity relationships, and provides examples of studies utilising each system”.
Notes: Highly cited article (> 92 citations 31/7/16, google scholar).
1.6 Mucosal immunisation: adjuvants and delivery systems
Citation: Moyle PM, McGeary RP, Blanchfield JT, Toth I. Mucosal immunisation: adjuvants and delivery systems. Current Drug Delivery 2004;1(4):385-96
Synopsis: This review aims to provide an overview of the immune system with respect to induction of mucosal immune responses, and provides an overview of a number of microbial (bacterial toxins, CpG DNA, cytokines / chemokines, live vectors, and virus like particles) and synthetic (microspheres, liposomes, and lipopeptides) strategies that have been investigated as adjuvants or delivery systems for mucosal vaccine development, with a focus on the delivery of vaccines via the oral route.
Notes: Highly cited article (> 76 citations 31/7/16, google scholar).