Papers by Dr Peter Michael Moyle
3.1 Synthesis of a highly pure lipid core peptide based self-adjuvanting triepitopic group A streptococcal vaccine, and subsequent immunological evaluation
Citation: Moyle PM, Olive C, Ho M-F, Good MF, Toth I. Synthesis of a highly pure lipid core peptide based self-adjuvanting triepitopic group A streptococcal vaccine, and subsequent immunological evaluation. Journal of Medicinal Chemistry 2006;49(21):6364-70
DOI: 10.1021/jm060475m
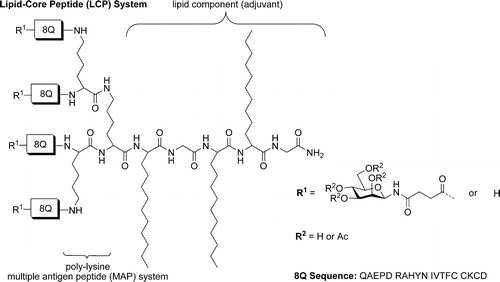
Synopsis: The development of peptide-based vaccines targeting group A streptococcus (S. progenes) is made difficult by the fact that there are over 200 different strains against which protection must be ensured. Thus, the use of a single peptide antigen is unlikely to protect against the majority of circulating strains. We have thus developed a synthetic approach that enables the defined conjugation of various peptide antigens to generate highly-pure, chemically-defined, multiepitope vaccines, which include the incorporation of a potent synthetic lipid adjuvant (lipid core peptide; LCP). This adjuvant allows our vaccine platform to be administered without any additional adjuvant, and because all of the vaccine components are in the one molecule, it ensures that all vaccine components are delivered to the same cell, significantly improving vaccine potency. The synthetic method is based on the native chemical ligation (NCL) reaction, and allows for vaccine production in excellent overall yield. Using this platform we demonstrate the ability to incorporate three group A streptococcal antigens into a candidate vaccine, and show that this vaccine is able to elicit high titres of serum IgG antibodies against each peptide antigen when administered to B10.BR (H-2K) mice
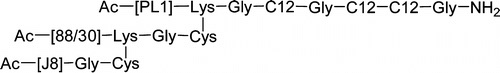
3.2 Toward the development of prophylactic and therapeutic human papillomavirus type-16 lipopeptide vaccines
Citation: Moyle PM, Olive C, Ho M-F, Pandey M, Dyer J, Suhrbier A, Fujita Y, Toth I. Toward the development of prophylactic and therapeutic human papillomavirus type-16 lipopeptide vaccines. Journal of Medicinal Chemistry 2007;50(19):4721-7
DOI: 10.1021/jm070287b
Synopsis: A library of synthetic human papillomavirus type-16 (HPV-16) vaccines incorporating: 1) four copies of the HPV-16 E7(44-62) antigen; 2) a lipid adjuvant (lipid core peptide;LCP); and 3) four copies of dendritic cell targeting mannose residues were produced by stepwise solid-phase peptide synthesis. The capacity for these vaccines to reduce/clear a HPV-16 tutor model (TC-1 cells) was assessed in C57BL/6 mice. Significant reductions in the size of TC-1 tumours was observed, with the incorporation of mannose resulting in a greater reduction in tumour size/number.